



Transportation:Ocean,Land,Air,Express,Others
Model No.: MFS-751
Place Of Origin: China
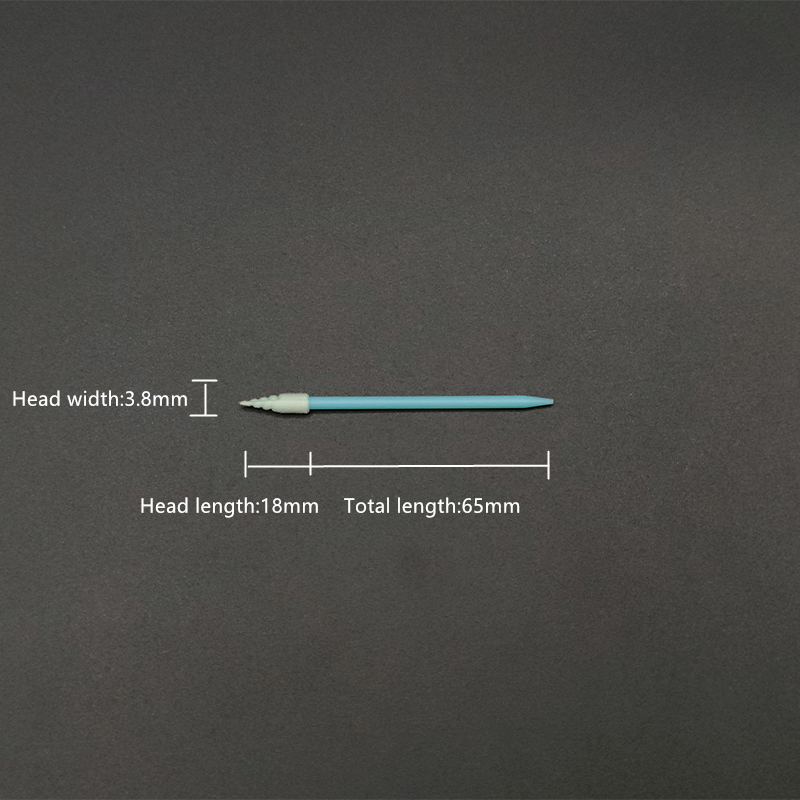
Total Length: 65mm
Tip Width: 3.8mm
Tip Thickness: 3.8mm
Tip Length: 18mm
Handle Width: 2.5mm
Handle Thickness: 2.5mm
Handle Length: 47mm
Packaging: Packing Details: 100pcs/bag
Transportation: Ocean,Land,Air,Express,Others
Cleaning Validation Requirements for Swabs
We may indeed have overlooked the requirement for swabs used for swab sampling, especially as more and more companies begin to use the TOC method for cleaning validation to determine residues, and the requirement for swabs is even more important.
At the beginning of the video, the TOC method cleaning verification requirements for Cleanroom Swabs are put forward, including the following four points:
Less background interference from TOC;
higher recovery rate;
lower particle production;
Sawtooth processing.
Lower TOC background interference, which needs no explanation but is often overlooked. It actually means, on the one hand, the TOC background on the cleanroom swab itself, and on the other hand, the TOC background in the water we use for swab sampling, as well as their consistency and reproducibility;
The higher recovery doesn't need explanation either, but is the recovery data from our hard-working swab sampling really valid? If the Cleanroom Swab is changing and the adsorption capacity of the cleanroom swab is different, is it reliable to use the constant recovery data to calculate? Does it make sense?
Particle generation can contaminate the area being wiped, which may subsequently contaminate the product. To reduce the risk of contamination, it is not that we use cleanroom swabs to wipe samples and then clean them, but to control the source of the cleanroom swabs used for wiping, and choose materials with fewer particles to fall off as cleanroom swabs.
These considerations can actually be reflected in our following documents:
Risk assessment and control of swab sampling effect;
Supplier selection requirements, acceptance and evaluation criteria of cleanroom swab for swab sampling;
Standard Practice for Swab Sampling
We typically describe the swab sampling procedure we require in the form of a picture in the swab sampling procedure for cleaning validation. The focus is on:
Uniform force, parallel one-way, covering the entire sampling area;
complete and reproducible;
We can see that this flat cleanroom swab is very suitable for swab sampling. And, here's the point - the video suggests two swabs for one sampling point:
cleanroom swab 1: wipe the front side horizontally first, and then wipe it vertically with the back side;
cleanroom swab 2: first wipe the front at a 45-degree angle to the left, and then wipe the reverse at a 45-degree angle to the right;
After wiping, the two cleanroom swabs were broken into a bottle, and then the solvent was added to dissolve the ingredients.
Of course, there are also larger package sizes.
In addition to considering the details that should be considered for each required item, how much care should it take to make all the items required for a sampling activity into a kit for easy use? !
A company with a small product range that has been able to do it for more than 50 years or more than a century, which does not need to focus on making the product to the extreme? And is this attitude worth learning?
You can also take a look at the cleaning video of a century-old German company that makes cleaning products to see how they make cleaning products and cleaning technology to the extreme:
In 1993, the U.S. Food and Drug Administration (FDA) issued testing guidance - Validation of Cleaning Procedures. Since then, provisions regarding cleaning procedures for pharmaceutical manufacturing environments, sampling and filling kits have received increasing attention. Initially, the need for cleaning validation was driven by cross-contamination of currently produced drug products by residues of active pharmaceutical ingredients (APIs) from previous run batches or cleaning agents from the cleaning process.
Any cross-contamination caused by foreign residues that poses a safety risk to the patient can result in depletion of the drug product. Because cross-contamination can cause changes in the potency, chemical properties, integrity and formulation of the drug. Therefore, equipment and work environments used in pharmaceutical manufacturing processes must be cleaned regularly and at regular intervals to prevent cross-contamination. These cleaning protocols must be validated in order to ensure that the purpose of cleaning is achieved - cleanliness that avoids cross-contamination.
In recent years, the development of cleaning validation has been continuously strengthened, and it is emphasized that the cleaning protocol should fully consider the safety of the drugs produced. At the same time, the growth of outsourcing and foreign pharmaceutical manufacturing has also strengthened the FDA's focus on cleaning procedures. Lack of documentation, training, and validation of cleaning procedures were among the top four issues for FDA Form 483 and Warning Letter issuances.
Swabs For Electronics Anti-static ESD Cleanroom Swab
MFS-751 is thermally bonded by 100PPI open-cell polyurethane foam & light green PP handle, its cleanroom laundered foam head possesses high solvent capacity and good cushioning. It is free from organic contaminants such as silicone, amide and phthalate esters, and it features very low non-volatile residue (NVR), ion content and particle generation. MFS-758 is designed with a small compact round handle and a thin flexible paddle head, and it’s the ideal swab for cleaning components in confined thin groove space where thickness height is the major concern.
Product Features
① Free from silicone, amide and phthalate esters
② Low in particles, ion content and non-volatile residue
③ High solvent capacity, soft and non-abrasive
④ Compatible with most common solvents such as IPA
⑤ Designed with flexible head paddle & long compact handle
Product Applications
① Cleaning with solvents such as IPA
② Cleaning surfaces and hard-to-reach areas
③ Remove flux residues and excess materials
Specification of Other Hot Selling Cleanroom Swab
| Model No. | Total Length | Tip Width | Tip Thickness | Tip Length | Handle Width | Handle Thickness | Handle Length |
| MFS-707 | 129mm | 17mm | 9.5mm | 26mm | 6.5mm | 2.7mm | 103mm |
| MFS-708 | 125mm | 19.5mm | 10mm | 27mm | 6.5mm | 2.7mm | 98mm |
| MFS-712 | 124mm | 13mm | 7.5mm | 25mm | 5.7mm | 2.5mm | 99mm |
| MFS-740 | 163mm | 6mm | 5.5mm | 17mm | 3mm | 3mm | 146mm |
| MFS-742 | 68.5mm | 3.6mm | 3.5mm | 11.5mm | 2.8mm | 2.8mm | 57mm |
| MFS-750 | 77mm | 3.2mm | 3.2mm | 11mm | 2.4mm | 2.4mm | 66mm |
| MFS-751 | 65mm | 3.8mm | 3.8mm | 18mm | 2.5mm | 2.5mm | 47mm |
| MFS-758 | 70mm | 3.2mm | 2.5mm | 10.5mm | 2.3mm | 2.3mm | 59.5mm |

Miraclean Technology Co, Ltd.
Established in 2003, Miraclean Technology is a manufacturer specializing in producing Cleanroom Products & Printers Cleaning Products. In order to meet or even exceed the industry standard, the plant is completely equipped with DI water system, sterilization equipment and purification workshops. Miraclean is committed to providing More Reliable Cleaning products for the contamination control industry. We are continuously striving for advancement to meet the demands of the growing market.


Packing and Shipping

Certificate

Exhibition
