



Transportation:Ocean,Land,Air,Express,Others
Model No.: MFS-708
Place Of Origin: China
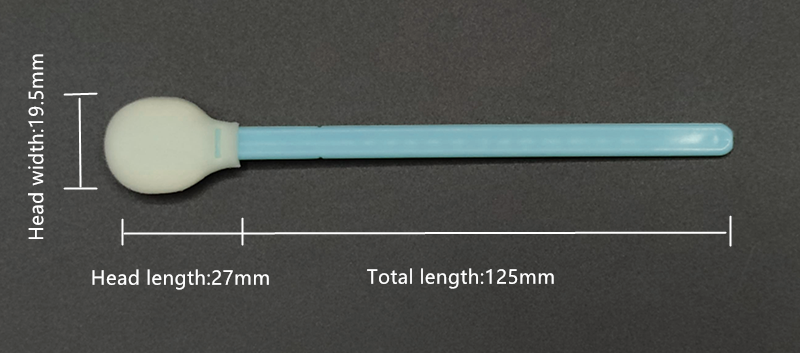
Total Length: 125mm
Head Length: 27mm
Head Width: 19.5mm
Head Thickness: 10mm
Handle Length: 120mm
Handle Width: 6.5mm
Handle Thickness: 2.7mm
Packaging: Packing Details: 100pcs/bag
Transportation: Ocean,Land,Air,Express,Others
Wipe Procedure - Precautions
cleanroom swab used for sampling are typically pre-wetted with water or other suitable solvent to remove surface residues. After pre-wetting and before sampling, it is very important to squeeze the side of the Cleanroom Swab into the inside of the vial to squeeze out the excess solvent, because the excess solvent itself will become residue, making the test result inaccurate. There is a direct physical interaction between swabs, solvent and residue; therefore, swab selection is critical to the effectiveness of the sampling process. The swabs used must meet the factors of ultra-low particle and fiber release, high liquid absorption and minimal leaching disturbance. Polyester swabs are specially treated to meet the toughest cleaning validation requirements for swabs.
The physical nature of the wiping process requires effective operator training prior to cleaning validation operations. Training minimizes the inherent subjectivity of the operator's sampling activities. Figure 1 shows the suggested wiping directions and movements for the actual wiping operation, which should be detailed in the training so that the operator can achieve a high degree of consistency. The double swab method can maximize the recovery rate, so this method is generally used.
Use a suitable extractant to release residues from the tip of the swab. Depending on the specific SOP for each region, the swab tip sample may need to be filtered or sonicated to extract as complete a residue as possible. The material quality of the swab tip and filter is critical for sample pretreatment. Any sampling tool that is not of the quality of a properly pretreated polyester swab will introduce additional foreign contamination for subsequent testing.
Cleaning Validation Procedures Standard procedures for cleaning validation are typically developed and validated by testing test slides in place of the equipment or other surfaces being cleaned. The choice of filters and extractants during sample preparation is also critical, as they have a large impact on recovery and can also affect extraction and filtration efficiency. Yang et al. have published the results of a systematic study on the effect of various solvent conditions and pH on recovery and filtration efficiency. If we choose an extraction solvent for a subsequent assay (eg HPLC) based solely on feeling, it may have some impact on filtration efficiency and recovery.
Residue Analysis - Analysis Considerations
The swab sampling in the cleaning validation program is to demonstrate that the cleaning operation is achieving the desired cleaning results. The best way to verify this effect (cleaning surfaces to avoid cross-contamination) is to test the recovery of known residues of the inoculum. This test method provides acceptable residue limits (RAL). Recovery is accomplished by some analytical test methods, typically HPLC (High Performance Liquid Chromatography) or TOC (Total Organic Carbon Test).
HPLC-UV systems generally have additional detectors, eg a mainframe (MS - for specific identification). It is important to realize early in the cleaning validation development process that recoveries are directly affected by the interaction of various variables introduced into the detection process with specific analytical detectors. The best approach is to study the impact of various variables in the cleaning validation process in advance, so that you can ensure that you fully understand the impact of these variables on the final recovery rate. If the detection verification method leads to abnormal detection results, it will be very troublesome to analyze and understand the results after a long period of time. Cleaning validation is a complex process requiring careful selection of sampling procedures and analytical methods. Therefore, in order to avoid abnormal test results that often occur in practice, we strongly recommend that only the highest quality swabs, filters and solvents are used during cleaning validation.
HPLC and TOC are very sensitive test methods for cleaning validation analysis. HPLC is a specific detection method because it can identify various peaks and determine what residues are responsible for; at the same time TOC is a commonly used non-specific test method for all organic carbon in a known environment. These methods are quantitative, so the typical analytical parameters of these methods must be evaluated, such as accuracy, precision, linearity, detectability, and limits of quantification.
HPLC is a very common test method in the pharmaceutical industry. In order to guarantee the complexity, sensitivity and importance of cleaning validation for drug safety, we need to pay special attention to the results of HPLC chromatographic analysis. At the same time, it is also important to avoid the use of materials that may introduce foreign contamination, which can interfere with the UV spectrum and detector.
In the event that such interference is unavoidable, we need to understand and eliminate such interference in order to ensure that cleaning validation procedures are compliant and scientifically supported at the time of inspection. In addition to the expected reasonable residual peaks in the chromatogram, any unexpected peaks need to be identified.
TOC (Total Organic Carbon) is a test method that measures the thermal conductivity and thus the relative carbon concentration. This method can measure the total, non-specific surface residue left over from the previous batch production. TOC tests are highly sensitive, typically detecting parts per billion (ppb, or µg/L) levels. Therefore, it is important to minimize the contamination of foreign organic carbon during swab sampling and sample preparation.
Generalize
In conclusion, cleaning validation is a critical step in cleaning the pharmaceutical environment. cleanroom swab are the best option for surface sampling during cleaning validation. These sampling and analysis methods have a direct impact on the recovery results obtained by HPLC or TOC analysis. At the same time, during the cleaning verification process, we must ensure that the relevant tool materials (such as cleanroom swab, filters, etc.) used are of the highest quality possible, so as not to release even trace levels of impurities that will affect the test results.

Foam Rubber Polyurethane Swab For Printhead Cleaning
MFS-708 is thermally bonded by 100PPI open-cell polyurethane foam & light green PP handle, its large circular head possesses excellent solvent-holding capacity and durability. It is free from organic contaminants such as silicone, amide and phthalate esters, and it features very low non-volatile residue (NVR), ion content and particle generation. MFS-708 is specifically designed for general purpose cleaning of curve surfaces, and it also could be used as the disinfectant swab for medical application.
Product Features
① Free from silicone, amide and phthalate esters
② Low in particles, ion content and non-volatile residue
③ High solvent-holding capacity, compact and non-abrasive tip
④ Compatible with most common solvents such as IPA
⑤ Designed with large rectangular head & firm handle
Product Applications
① Cleaning with solvents such as IPA
② Cleaning large surfaces, printheads, equipments
③ Remove flux residues and excess materials
Specification of Other Hot Selling Cleanroom Swab
| Model No. | Total Length | Tip Width | Tip Thickness | Tip Length | Handle Width | Handle Thickness | Handle Length |
| MFS-707 | 129mm | 17mm | 9.5mm | 26mm | 6.5mm | 2.7mm | 103mm |
| MFS-708 | 125mm | 19.5mm | 10mm | 27mm | 6.5mm | 2.7mm | 98mm |
| MFS-712 | 124mm | 13mm | 7.5mm | 25mm | 5.7mm | 2.5mm | 99mm |
| MFS-740 | 163mm | 6mm | 5.5mm | 17mm | 3mm | 3mm | 146mm |
| MFS-742 | 68.5mm | 3.6mm | 3.5mm | 11.5mm | 2.8mm | 2.8mm | 57mm |
| MFS-750 | 77mm | 3.2mm | 3.2mm | 11mm | 2.4mm | 2.4mm | 66mm |
| MFS-751 | 65mm | 3.8mm | 3.8mm | 18mm | 2.5mm | 2.5mm | 47mm |
| MFS-758 | 70mm | 3.2mm | 2.5mm | 10.5mm | 2.3mm | 2.3mm | 59.5mm |

Miraclean Technology Co, Ltd.
Established in 2003, Miraclean Technology is a manufacturer specializing in producing Cleanroom Products & Printers Cleaning Products. In order to meet or even exceed the industry standard, the plant is completely equipped with DI water system, sterilization equipment and purification workshops. Miraclean is committed to providing More Reliable Cleaning products for the contamination control industry. We are continuously striving for advancement to meet the demands of the growing market.


Packing and Shipping

Certificate

Exhibition

Miraclean provide reliable products and custom design services to customers from all over the world, any requires about cleanroom swab price,polyurethane swab price shenzhen,Printhead Swab price china,Polyester Wipes best price,Non-woven Wipes price,Cleanroom Wipes cost-effective please contact us.